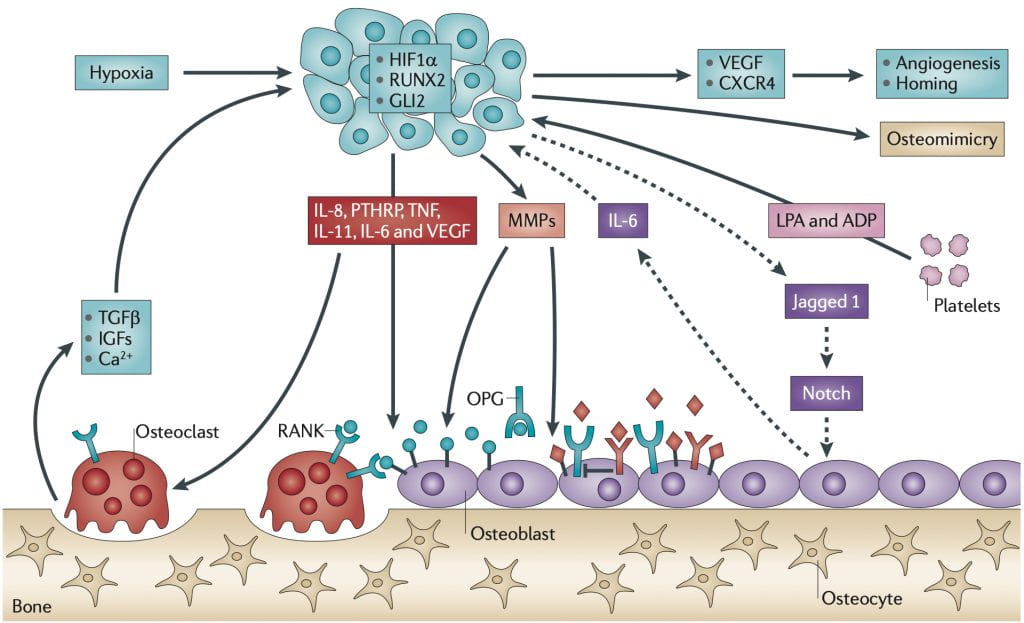
Mechanisms of tumour-associated bone loss
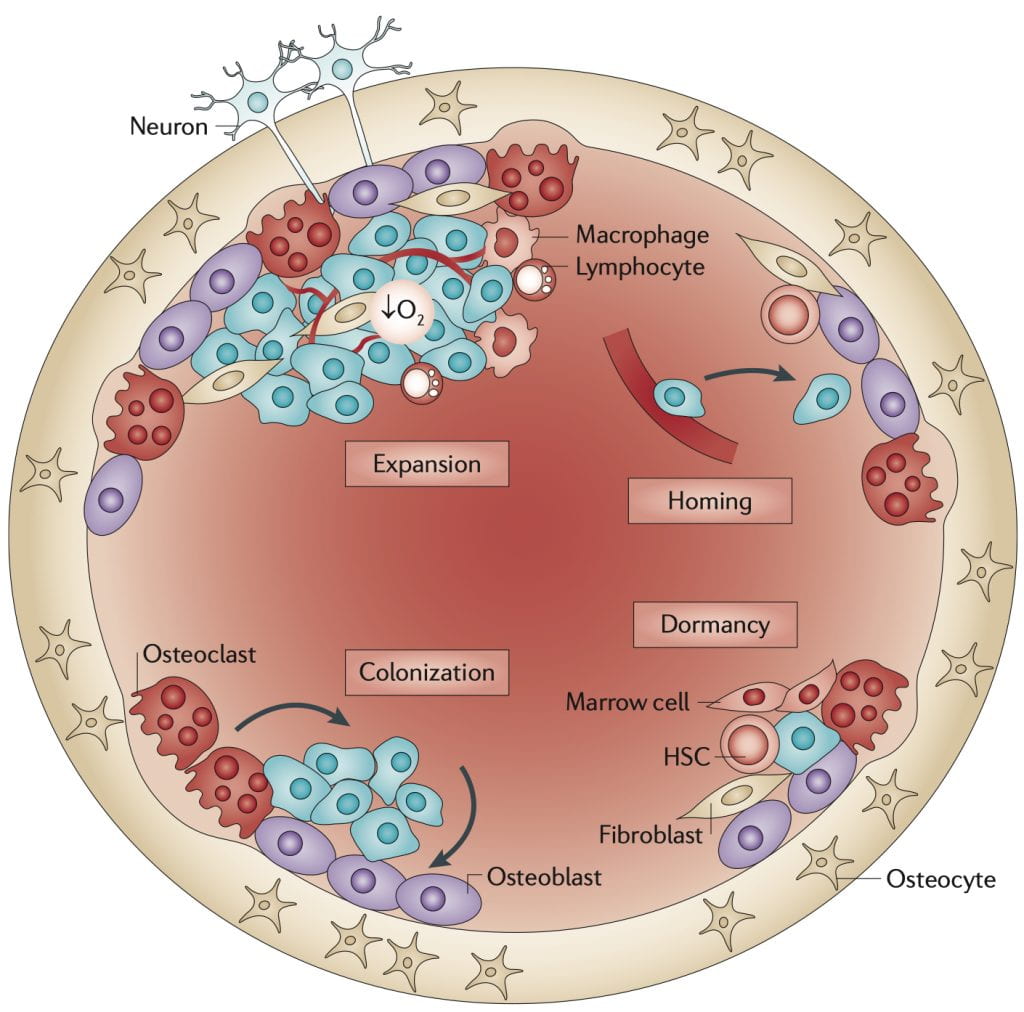
Cross-section of bone depicting stages of bone metastases
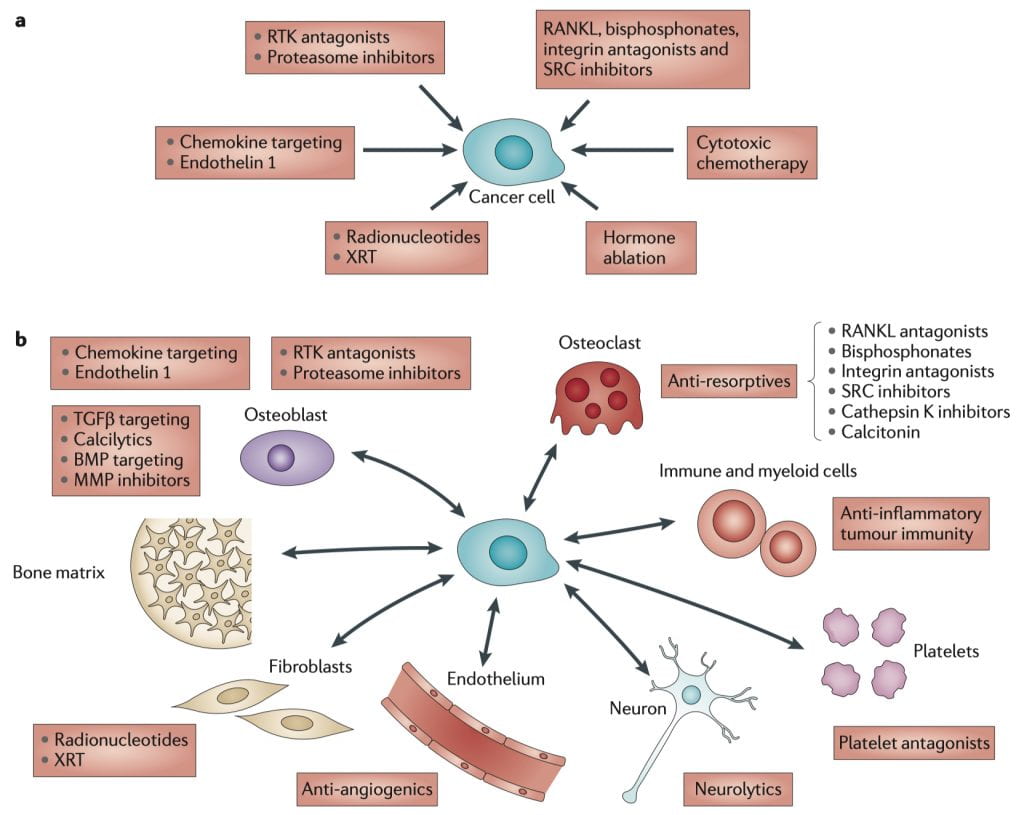
Overlapping benefits of targeting tumour and stromal cells for bone metastases
Research Interests
The skeleton is the organ most commonly affected by metastatic cancer in humans. Mechanisms by which skeletal metastases are established are unclear; however, osteoclast activation plays a critical role in the pathogenesis of bone metastases. Our laboratory focuses on the molecular mechanisms through which tumor cells metastasize to bone. We are specifically interested in characterizing ways in which host cells within the tumor microenvironment (including osteoclasts, osteoblasts, stromal cells, and immune cells) influence tumor growth. Bone-metastatic tumor cells are known to secrete molecules that affect the activity of these host cells. In turn, host-derived growth factors can further enhance tumor growth, known as ‘the vicious cycle’. Understanding this will facilitate the identification and development of host-targeted therapeutics that will decrease tumor growth and tumor-associated bone disease.
Research Projects
The integrin β3 subunit (ITGB3) has been implicated in the development of metastases because of its critical role in osteoclastic bone resorption (αvβ3), and its role in platelets in tumor cell homing (αIIbβ3). In mice model of metastasis, Itgb3-/- mice have decreased bone metastasis and tumor associated bone loss. Integrin beta3 is critical for tumor invasion, neoangiogenesis, and inflammation making it a promising cancer target. However, preclinical and clinical data of integrin beta3 antagonists have demonstrated no benefit or worse outcomes. Our data uncovered an anti-tumoral role for integrin beta3 signaling in tumor associated macrophages (TAMs). In mice orthotopic tumor model, ITGB3 negatively regulates pro-tumor immune suppressive M2 macrophage polarization and function. Our animal model results suggest that pro-tumor macrophages play a key role in resistance to ITGB3 antagonists in patients with cancer. We found that integrin beta3 signaling controls the balance between anti-tumor and pro-tumor immune cells through effects on STAT6/STAT1 signaling, which in part explains the mixed results of integrin antagonists in the clinic. Our findings highlight the important role of TAMs when designing clinical trials with integrin beta3-targeted treatments in cancer. The long-term goal of this project is to understand the mechanism of ITGB3-mediated macrophage polarization and immune suppression at each stage of tumor progression and metastasis.
Bone metastases occur in approximately 70% of metastatic breast cancer patients, often leading to the development of severe skeletal injuries. In an attempt to improve drug efficacy against metastases at this difficult-to-treat location, we evaluated whether targeted nanoparticle-mediated drug delivery against integrin αvβ3 could improve chemotherapy efficacy against bone metastases. We observed that αvβ3-targeted micelle nanoparticles (αvβ3-MPs, ~12.5nm) specifically colocalized with murine breast cancer bone metastases. Treating mice bearing bone metastases with αvβ3-MP-mediated delivery of the chemotherapeutic docetaxel significantly reduced bone tumor burden and bone destruction with less hepatotoxicity, as compared to treatment with an equal dose of free-docetaxel. This work provides support for enhanced drug delivery to breast cancer cells within bone by exploiting tumoral expression of integrin αvβ3. We are now evaluated whether we can also use these nanoparticles to target the tumor-supportive immune system, in addition to targeting the tumor cells.
Integrin expression by tumor cells can mediate enhanced invasion, metastasis, and resistance to standard therapies. We have shown that integrin β3 is upregulated on breast cancer bone metastases in both mice and humans, and that specific delivery of chemotherapy to β3-expressing cells can attenuate tumor burden even though systemically administered therapy fails. This suggests a possible role for integrin β3 as both a promoter of therapy resistance in the bone and as an exploitable target for killing cells that ordinary treatments fail to affect. We are currently characterizing the mechanisms through which integrin β3 mediates therapy resistance in bone-resident cells and developing regimens that combine systemic and targeted treatments to eradicate resistant cells and forestall relapse.
Targeted therapies increase drug delivery to tumor and host cells and reduce side effects. An in depth understanding of target molecule expression is required for specific drug delivery. We are currently identifying target molecules for metastatic breast cancer through patient derived xenograft (PDX) mouse models and patient tissue samples. This work directly informs ongoing pre-clinical studies and phase I clinical trial design for novel targeted therapies.
Adult T-cell leukemia/lymphoma (ATL) develops in a subset of patients infected with the retrovirus HTLV-1. Osteolytic bone lesion and hypercalcemia are common complications in patients with ATL. Expression of HTLV-1 HBZ oncogene, under the regulation of the granzyme B promoter, results in lymphoproliferative disease and bone loss. To investigate the role of HBZ in the development of hematopoietic disease and bone loss, we are currently using humanized mouse model as a novel infectious model that faithfully mimics all stages of pathogenesis from HTLV-1 infection to ATL development.